ni2+ unpaired electrons|Ni2+ Electron Configuration(Explained for Beginners) : Clark We first need to find the number of electrons for the Ni atom (there are 28 electrons) using the Periodic Table. When we write the configuration, we'll put all 28 electrons in orbit .more. To. 3 Balikan Halina’t balikan ang nakaraang aralin na makakatulong sa iyo upang maiugnay mo ang mga nakaraang leksyon sa kasalukuyan. Panuto: Basahin ang mga pahayag. Kapag tama ang pahayag, lagyan ng tsek (/) ang bilang at ekis (x) kung mali. Ilagay ang iyong sagot sa kuwaderno.
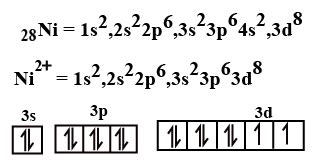
ni2+ unpaired electrons,Electronic configuration of Ni + 2 = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 8 electrons is removed from the lower energy shell. Ni + 2 has 8 electrons in its outermost shell out of which there are six are paired electrons and two unpaired electrons. The magnitude of the magnetic moment can be calculated by the formula, [n(n+2)] 1/2, where n is the number of unpaired electrons. For Ni 2+ the number of .
We first need to find the number of electrons for the Ni atom (there are 28 electrons) using the Periodic Table. When we write the configuration, we'll put all 28 electrons in orbit .more. To.
0. C. 8. D. 2. Solution. Verified by Toppr. Nickel has two unpaired electrons in the 3d sublevel. When nickel ionizes to the +2 state it loses the two electrons in the 4s, so .
How many unpaired electrons are present in N i2+? A. 0. B. 2. C. 4. D. None of the above. Solution. Verified by Toppr. N i2+ looses electrons from the 4s orbital and has 8 .
Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. After removal of two electrons from outermost shell the electron .
Ni2+ ion consists of 2 numbers of unpaired electrons. The atomic number of the Nickel element is 28. And the plus two (+2) charge on the nickel indicates that it has .Ni2+ Electron Configuration(Explained for Beginners) How many unpaired electrons are present in N i+2? A. 0. B. 2. C. 4. D. 8. Solution. The correct option is B. 2. The ground state electronic configuration of Nickel is: [Ar]3d84s2. .
How many unpaired electrons are there in Ni 2+? A. 0. B. 2. C. 4. D. 8. Medium. Solution. Verified by Toppr. Correct option is B) As we know, electronic configuration of Ni .
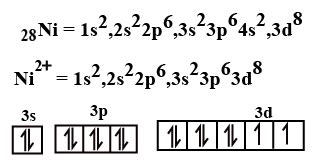
How many unpaired electron (s) are there in N i2+ ion if the atomic number of Ni is 28. View Solution. Q 5.1 Answer. Junaid Mirza. Jun 10, 2018. 1s2 2s2 2p6 3s2 3p6 4s0 3d8. Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. After removal of two electrons from outermost shell the electron configuration of Ni2+ is. .
Structure of the octahedral ferricyanide anion. Because the overall charge of the complex is 3-, Fe is in the +3 oxidation state and its electron count is 3d 5. The same procedure can be applied to any transition metal complex. For example, consider the complex [Cu (NH 3) 4] 2+. Because ammonia is a neutral ligand, Cu is in the 2+ oxidation .Cr3+. Solution. Verified by Toppr. V 3+ has 3d2 outer electronic configuration with 2 unpaired electrons. N i2+ has 3d8 outer electronic configuration with 2 unpaired electrons. Thus, V 3+ and N i2+ have same number of unpaired electrons. Was this answer helpful?
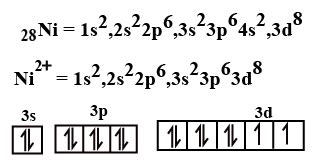
Exploring the Ni 3d Orbital Unpaired Electrons Induced Polarization Loss Based on Ni Single-Atoms Model Absorber. Hongsheng Liang, Hongsheng Liang. MOE Key Laboratory of Material Physics and Chemistry under Extraordinary, School of Physical Science and Technology, Northwestern Polytechnical University, Xi'an, 710072 P.R. .
Solution. The correct option is C 6. The electronic configuration of Cr is 1s2 2s2 2p6 3s2 3p6 3d5 4s1. Half filled orbitals have a more stable configuration. Number of unpaired electrons is 6. Suggest Corrections. 55.
ni2+ unpaired electrons Recall, that diamagnetism is where all the electrons are paired and paramagnetism is where one or more electron is unpaired. This property can be used to determine the magnetism and in some cases the filling of the orbitals. For example, given a high spin octahedral molecule, one just has to fill in all the orbitals and check for .
V. 2. +. which ion contains more number of unpaired electrons? Solution. Verified by Toppr. T i2+ has electronic configuration;[Ar]3s2. V 2+ has electronic configuration:[Ar]3s23d1. Therefore T i2+ does not have any unpaired electron and V 2+ have one unpaired electron .ni2+ unpaired electrons Ni2+ Electron Configuration(Explained for Beginners) To find: We have to find the number of unpaired electrons. Solution: The given ion is . Ti is a transition element. It has the atomic number 22. When two electrons are removed from Ti the atomic number becomes 20. Thus the electronic configuration of the ion is as follows-Thus, the 3d orbital contains two unpaired electrons.Ni2+ and Unpaired Electrons Ni2+ is a transition metal ion with a d8 electron configuration. This means it has eight electrons in its d orbitals, and in the absence of ligands, these electrons would pair up in the lower energy levels. However, when Ni2+ is coordinated to ligands, the crystal field splits the d orbitals into two sets of energy .
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: 5. Which has the greatest number of unpaired electrons in the ground state? A. Cr3+ B. Mn2+ C. Ni2+ D. Ni E. V2+. Show transcribed image text. There are 3 steps to solve this one. Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.How many unpaired electron (s) are there in N i2+ ion if the atomic number of Ni is 28. View Solution. Q 4. Which of the following has the maximum number of unpaired electrons? ( Given Atomic number: Sc =21,F e =26,V =23,N i =28) View Solution. Q 5. Atomic number of M n,F e,Co andN i are 25,26,27,28 respectively.The correct option is A Only Zn2+ is colourless and N i2+,Cu2+ and Cr2+ are coloured. Zn2+(3d10) has zero unpaired electron (colourless). N i2+(3d8) has 2 unpaired electrons (coloured). Cu2+(3d9) has 1 unpaired electron (coloured). Cr2+(3d4) has 4 unpaired electrons (coloured).
How many unpaired electrons are present in Gd(Z = 64) ? View Solution. CENGAGE CHEMISTRY-ATOMIC STRUCTURE-Exercises Single Correct. For a given principal level n = 4 the energy of its subshells is of t. 01:10. Sodium choride impqarts a yellow colour to the Bunsen flame .This ca.The electron configurations of atoms follow a standard notation in which all the atomic subshells that contain electrons (with the number of electrons they hold written in superscript) are placed in a certain sequence. For example, the atomic number of Sodium is 11 and its electron configuration is 1 s 2 2 s 2 2 p 6 3 s 1. Aufbau principle:- Write down the electronic configuration of Fe3+ and Ni2+. How many unpaired electrons are present? (Given, Atomic number, Fe = 26, Ni = 28) . Write the electronic configuration of the following and report the number of unpaired electron in each a. `Mn^(2+)` b.`Cr^(2+)` c`Fe^(2+)` d.`Ni^(2+)` asked May 24, 2019 in Chemistry by .Step 2: Calculating the unpaired electrons. In Phosphorus, the p-subshell has 3 electrons in it. Now we know that these electrons will fill the orbitals according to Hund's rule of maximum multiplicity. That is the orbitals will first be filled singly and after all the orbitals are filled singly, then the pairing of the electrons will start.
ni2+ unpaired electrons|Ni2+ Electron Configuration(Explained for Beginners)
PH0 · What is the electron configuration of #Ni^(2+)#?
PH1 · Ni2+ Electron Configuration(Explained for Beginners)
PH2 · How many unpaired electrons are there in Ni^{2+} (Z = 28) ?0842
PH3 · How many unpaired electrons are there in Ni2+? Q&A
PH4 · How many unpaired electrons are there in Ni ^2
PH5 · How many unpaired electrons are present in Ni2+?
PH6 · How many unpaired electrons are present in Ni +2 ?A. 0B. 8C.
PH7 · How Many Unpaired Electrons Are In Ni2+
PH8 · Electron Configuration for Nickel (Ni and Ni2+, Ni3+ ions)
PH9 · Electron Configuration for Ni, Ni2+, and Ni3+ (Nickel